|
The Dating Game data | hook | main | background & resources | student Hillary Tulley Niles North High School Skokie, Illinois tulley@tea.rice.edu Adapted from Darnell Giron M&M half-life in Earth at Hand A collection of arcticles from NSTA's journals. Collectd by Sharon M. Stroud and Jeffrey C. Callister Overview Grade Level/Discipline National Standards Materials Engagement and Exploration (Student Inquiry Activity) How far from the ocean are the seals? How can we find out? - Students will probably have a good idea how to do this. Invite a few students to calculate this using a map of Antarctica or of the Dry Valleys. How old do you think these seals might be? How can we find out? How do we know? Now is the time to introduce the concept of absolute dating and half- life.(See Background). Distribute student sheets and graph paper. Go over student directions - sheets follow. Before going into lab, students will make a data table, using a ruler and pencil. Elicit suggestions from the class as to what needs to be in the data table:
Part of the fun of this lab is eating the M&M's. Students can eat the daughter or "changed" M&M's. Encourage equitable sharing. However, if this is not something that you want to encourage, you can use pennies instead. After students have completed the lab and recorded data in the tables, it is time to produce the graphs. Again, elicit from students what things need to be included in a graph.
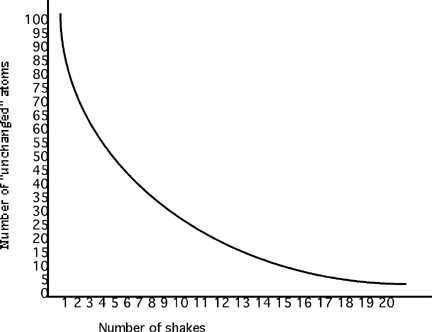
Explanation (Discussing) Which kind of the M&M's would be called parent? The "unchanged" Which would be the daughter? The "changed." Relate this to radioactive atoms. Have them explain how they think scientists could use radioactive decay to determine time since an organism's death. Design a way for all students to demonstrate understanding by this point. How many "shakes" old would an organism be that had 15 "unchanged" atoms left? If we convert the shakes to years, could we use this to tell time? Elaboration (Polar Applications) Use these assignments to see if the students can transfer what they should have learned to a new situation. The situation presented is based on a true finds but have been embellished to make more useful scenarios. Teacher Discovers Death in Wright Valley, Antarctica Students will be ready and eager to discover the age of the mummified seal from Wright Valley. Show the picture of the seal again and tell students that they will be able to determine the age of the seal by using the decay of carbon isotopes. Eventually the scientists requested a 14C radioactive dating test done on the seal's body. To their astonishment the test found that for every 100,000,000 12C atoms present in the seal's body,4,100 radioactive 14C atoms were present, instead of the 10,000 atoms expected for a recently deceased seal. How long ago did the seal die? _________________________. If your class can do it, give them the minimal information required: 14C half-life is approximately 5,700 years and let them create their own table, graph and solution to the problem. Some classes may need the following table, but all can generate the 14C graph on their own. Radioactive Decay of Carbon-14
II. Murder Above the Arctic Circle The body of a man wearing the traditional clothes of the Saami people was found at the bottom of one of the many peat bogs that remain from the last glacial retreat. Buried in the back of his skull was a stone ax, made in the archaic style. The stone ax head was sharpened by chipping and the blade was bound to a forked wooden handle by crisscrossed hide strips. Bog acid has tanned the man's skin. Although his skin is wrinkled and tightly drawn over his facial bones, his features are still distinguishable and his clothes and internal organs are still intact and available for police analysis. The withered condition of the body has convinced the police that the homicide happened at least 10 yeas ago. From forensic evidence, we can conclude that he was murdered in a different place, dragged to the bog and tossed in. After months of investigation no new evidence or information was turned up Eventually the police requested a 14C radioactive dating test done on the victim's body and clothes.To their astonishment the test found that for every 100,000,000 12C atoms present in the man's body, and clothes only 3,000 radio-active 14 C atoms were present, instead of the 10,000 atoms expected for a recently deceased person. This has greatly confused the police who had assumed that the murder was a recent event. How long ago did the murder take place ?_________________________. If your class can do it, give them the minimal information required: C14 half-life is approximately 5,700 years and let them create their own table, graph and solution to the problem. Some classes may need the following table, but all can generate the C14 graph on their own. Radioactive Decay of Carbon-14
Exchange (Students Draw Conclusions) Evaluation (Assessing Student Performance) data | hook | main | background & resources | student |