
|
|
Carbon Dioxide: The Heat is On
Overview
Greenhouse gases are those that prevent the radiant energy of Earth from
escaping our atmosphere. Carbon dioxide is needed for life here on Earth,
but it one of those gases labeled as causing the greenhouse effect.
Usually carbon dioxide given off from respiration and the carbon dioxide
used by photosynthesis are in balance, but the burning of so many fossil
fuels (coal, gas, gasoline) is causing an increase in the release of carbon
dioxide. These two activities, The Heat is On and Smog Be Gone, explore
the effect of greenhouse gases on heat absorption in the atmosphere and the
effect of the biological processes of phytoplankton on these greenhouse
gases. These two activities may be used after the activity on Secret
Agents of Dissolved Oxygen, which establishes the effects that
phytoplankton have on the chemical properties of water.
Rationale
Phytoplankton have a key role in the production of oxygen and the
resulting use of carbon dioxide in the atmosphere during photosynthesis.
They alter the chemical characteristics of the water due to their
biological processes. Because of the short season of light availability
in Antarctica, the phytoplankton are profoundly more productive in each
24 hour period than are general oceanic phytoplankton. Therefore,
the waters of Antarctica have an important role as a significant carbon
dioxide sink. This may prove to be extremely important in light of our
greenhouse gases problems.
Grade Level/Discipline
9-12, but may be adapted to advanced 8th grade classes
Objectives
- Students will observe the effect of the gases resulting from a burned
fossil fuel on the heat absorption of air.
- Students will determine the ability of phytoplankton to remove
greenhouse gases from the atmosphere
**See Teacher Background for alternatives to probe-ware measuring
dissolved oxygen, temperature, and carbon dioxide
National Standards
Teaching Standards: A, B, D, E; Content Standards: A, C, F;
Program Standards: B; System Standards: A, B, F
Teacher Preparation for
Activity
Materials
For each group:
- 2 large jars with screw lids that have holes for probe-ware or
thermometer
- - pickle jars from the cafeteria work well
- 2 temperature probes or thermometers
- 2 CO2 probes or limewater Calcium oxide (CaO)
- 2 candles
- matches or lighter
- 1 sunlamp
Directions for limewater
- Slowly add calcium oxide (CaO),one teaspoon at a time, to a large Pyrex
beaker of water. If you add too rapidly, the whole container could boil
over, so do a small bit at a time. It will be hot, so handle with
insulated gloves or beaker tongs.
- Continue adding CaO to the solution until no more will dissolve.
- Make up solution right before use and keep tightly closed because it
will react with the CO2 from the air, turning it prematurely cloudy.
Directions to use limewater
- Quickly add 20ml limewater to the bottom of the jars after the candle
has been burned and place the lid or cork back
- Shake or agitate the bottle so that the CO2 in the bottle will mix with
the limewater.
Pre-activity set-up
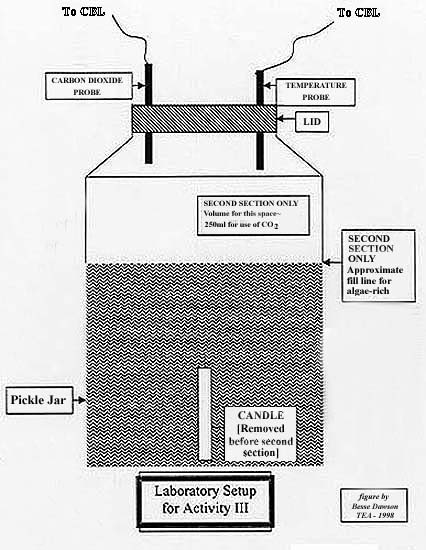
- See Figure I for jar setup
- Place one sunlamp fairly close to and the same distance from the two
jars.
Time Frame
Teaching Sequence
Engagement and Exploration (Student Inquiry Activity)
- Divide the students into lab groups or set up the activity as a
demonstration.
- Hand out Data Collection Sheet SM - 3
- Inquire of the students the purpose of the second jar (control). Why
is it a control?
- Ask the students what the purpose of the sunlamp is this time (heat).
- Assign one student in each group to be a time-keeper so that accurate
time intervals can show the increase and eventual decrease in temperature
in a controlled manner.
Explanation (Discussing)
- Ask the students to post their data and average each groups findings.
- Ask what function the burning candle served in the activity. (fossil
fuel burning)
- How did the burned hydrocarbon affect the heat retention of the
atmosphere?
- How can this increase in carbon dioxide affect our global weather
patterns? (warming trend)
Elaboration (Polar Applications)
How could the global warming affect us? (disruption of weather patterns,
melting of temperate glaciers, which cause faster ice shelf breakup, which
causes faster ablation of the ice sheets, which results in greater,
significant sea level rise, flooding coastal areas)
Exchange (Students Draw Conclusions)
Evaluation (Assessing Student Performance)
Authors
Besse Dawson
Pearland High School
3775 S. Main Street
Pearland, Texas 77581
Background
To show the effects of burning fossil fuels on the atmosphere, a
simple activity involving a candle burned inside a large, clear jar with
temperature and CO2 probes inserted Another large pickle jar with the
same probes filled with regular atmospheric air serves as a control. A
sunlamp is directed on both jars and both CO2 and temperature are recorded
for 30-45 minutes. The lamp is shut off and the CO2 and temperature are
again recorded for 30-45 minutes.
If a CO2 probe is not available, using the limewater will show the
presence of CO2 by turning cloudy. The limewater absorbs 25-35% of its
weight in carbon dioxide, forming calcium carbonate which is a white
precipitate. Be aware of the safety concerns:
Strongly corrosive and irritating to skin, mucous membranes and
eyes. Ingestion can cause severe damage to the G.I. tract.
Activity III should be used after this.
Resources
Consultants:
Dr. Dave Karl Principal Investigator/Research Mentor University of Hawaii dkarl@soest.hawaii.edu
Chris Carillo University of Hawaii
Dr. Nancy Bell University of Texas Medical Branch
Dr. Virginia Gordy Pearland High School
Biological Oceanographic Processes 3rd Edition; T.R. Parsons, M. Takashi,
B. Hargrave; Butterworth-Heineman, 1995.
Student Reproducible Masters
Carbon Dioxide The Heat is On!
Group Names ______________________________________________________
Procedure:
- Set up two large jar as shown in the picture on accompanying page.
- Light the candle in the second one and let it burn for five minutes.
- Blow out the candle and quickly tightly shut the lid to trap the smoke.
- Shine a sunlamp on both the jars so that both are equally heated by the
lamp.
- Record the temperature and carbon dioxide values every 5 minutes for a
total time of 30 minutes.
- Turn off the lamp and remove it from the area close to the jars.
- Record the temperature every 5 minutes for the next 30 minutes.
Time 5 10 15 20 25 30
T CD T CD T CD T CD T CD T CD
Heating
Jar # 1
(plain jar)
Heating
Jar # 2
(candle)
Cooling Jar
# 1
Cooling Jar
# 2
Graph the data using a different color for the control jar values and candle
jar values.
Analysis
1. What happened to the temperatures of the plain jar versus the candle
jar.
2. What is the impact of the candles burning on the heat retention?
CONCLUSION:
1. What could cause similar products being put in the atmosphere?
2. What impact could placing these products in our atmosphere have on it?
SM 3
Figure 1
We
look forward to hearing from you! Please review this activity.
Return to top of
page
Back to: TEA
Activities Page
|


