
|
|
Smog Be Gone
Overview
Greenhouse gases are those that prevent the radiant energy of
Earth from escaping our atmosphere. Carbon dioxide is needed for
life here on Earth, but it one of those gases labeled as causing
the greenhouse effect. Usually carbon dioxide given off from
respiration and the carbon dioxide used by photosynthesis are in
balance, but the burning of so many fossil fuels (coal, gas,
gasoline) is causing an increase in the release of carbon
dioxide. These two activities, The Heat is On and Smog Be Gone,
explore the effect of greenhouse gases on heat absorption in the
atmosphere and the effect of the biological processes of
phytoplankton on these greenhouse gases. These two activities
may be used after the activity on Secret Agents of Dissolved
Oxygen, which establishes the effects that phytoplankton have on
the chemical properties of water.
Rationale
Phytoplankton have a key role in the production of oxygen and the
resulting use of carbon dioxide in the atmosphere during photosynthesis.
They alter the chemical characteristics of the water due to their
biological processes. Because of the short season of light availability
in Antarctica, the phytoplankton are profoundly more productive in each
24 hour period than are general oceanic phytoplankton. Therefore,
the waters of Antarctica have an important role as a significant carbon
dioxide sink. This may prove to be extremely important in light of our
greenhouse gases problems.
Grade Level/Discipline
9-12, but may be adapted to advanced 8th grade classes
Objectives
- Students will observe the effect of the gases resulting from a burned
fossil fuel on the heat absorption of air.
- Students will determine the ability of phytoplankton to remove
greenhouse gases from the atmosphere
**See Teacher Background for alternatives to probe-ware measuring
dissolved oxygen, temperature, and carbon dioxide
National Standards
Teaching Standards: A, B, D, E; Content Standards: A, C, F;
Program Standards: B; System Standards: A, B, F
Teacher Preparation for
Activity
Extension:
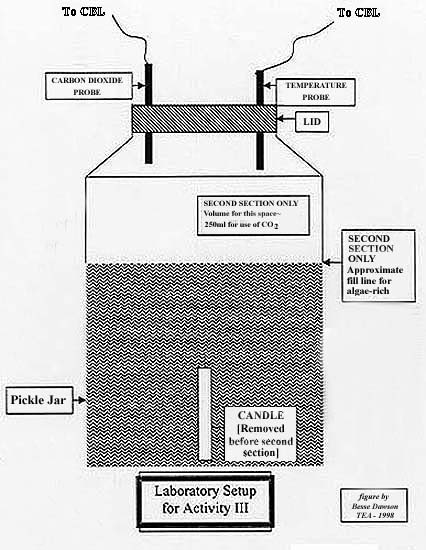
- Students should use the same setup as Activity II with the exception of
the removal of the candle (and limewater
solution if used) and the addition of algae-rich water. See
Figure 1
- Use SM-4
- Ask what things put carbon dioxide in the air. What good is the carbon
dioxide? (used in photosynthesis) What harm can it do? (greenhouse effect
is strengthened)
Materials
- Algal culture
- *Directions: Add liquid plant food in the amount given for
hydroponic growth to live (pond or aquarial) water. Expose solution to
intense sunlight and/or sunlamp. Water should be green, indicating an algal
bloom.
- 2 large jars with screw lids - Cafeteria pickle jars work well
- pH testing probe or pH testing kit, such is obtained from a pool supplier
- temperature probe or thermometer
- Carbon dioxide probe or limewater
Pre-activity set-up
- This activity may take one to two weeks as carbon dioxide uptake is
slow. It is also necessary to make certain that the water used in the jar
is very green with algae to maximize the impact.
- See Figure 1 for general setup.
- Use SM-3
- Carbon dioxide readings should be taken at the onset of the experiment
and once every day to monitor progress.
Teaching Sequence
Engagement and Exploration (Student Inquiry Activity)
- This activity is best done as a single demonstration unless you have
lab space to accommodate a long-term project for multiple classes. This
project can take from 1 to 2 weeks to have clear results.
- Hand out SM - 4 to students to record the results on a daily basis.
- The setup needs to be in strong natural or sunlamp light.
Explanation (Discussing)
Elaboration (Polar Applications)
- After the results have been obtained, students should discuss what
happened and why.
- Have the students come to a consensus on what occurred in the jars. (
Photosynthetic processes used excess carbon dioxide in the jar.)
- Tie in the difference in cyanobacteria from Antarctica as opposed to
that in more temperate climates or those that have more consistent
daylight. See Rationale at the beginning of these activities.
- Let the students brainstorm on how this knowledge could be used to
alleviate the increase in burning of fossil fuels that we have incurred
this century.
Exchange (Students Draw Conclusions)
Evaluation (Assessing Student Performance)
Authors
Besse Dawson
Pearland High School
3775 S. Main Street
Pearland, Texas 77581
Background
Resources
Consultants:
Dr. Dave Karl Principal Investigator/Research Mentor University of Hawaii dkarl@soest.hawaii.edu
Chris Carillo University of Hawaii
Dr. Nancy Bell University of Texas Medical Branch
Dr. Virginia Gordy Pearland High School
Biological Oceanographic Processes 3rd Edition; T.R. Parsons, M. Takashi,
B. Hargrave; Butterworth-Heineman, 1995.
Student Reproducible Masters
Smog Be Gone!
Group Names ______________________________________________________
Procedure:
- Use the same set-up as The Heat is On. Remove candle.
- Place algae-rich water in the bottom 2/3 of one jar and plain water in
the bottom of the second jar.
- Put the jar lids back on with the probes for temperature and carbon
dioxide inserted.
- Take readings once a day for 10 to 14 days.
Bottle w/algae Bottle w/plain water
DAY Temperature Carbon Dioxide Temperature Carbon Dioxide
1
2
3
4
5
6
7
8
9
10
11
12
13
14
Analysis
1. Graph the data using color coding for the 2 jars.
2. What happened to the temperature values in each jar?
3. What happened to the carbon dioxide in each jar?
CONCLUSION:
1. In what way did the algae-rich water affect the temperature and/or the
carbon dioxide levels?
2. What application might this have to helping our problem with global
warming?
SM-4
Figure 1
We
look forward to hearing from you! Please review this activity.
Return to top of
page
Back to: TEA
Activities Page
|


