
|
|
29 August, 2003
Romans and Ozone
Well, it would not be right if I did give a short lesson on Ozone! After all, the loss of ozone in our atmosphere is the reason I am in Antarctica in the first place.
I like ice cream, and so did the Romans, but making things cold back then was a lot trickier than it is now. In those times they would cart packed snow from the mountains and make a fruit sorbet for the richest people in town. This decadence of cold foods (cold drinks were once the rage for the nobility of France) has practical importance. Keeping food cold is a way to preserve it. Your great grandparents may still tell stories of the ice wagon, as most people before 1920 preserved their food with ice blocks cut from ponds and lakes. So it is not surprising that new ways to refrigerate food where developed. The early refrigerators used Alcohol in the coils that cool the interior of the refrigerator. The alcohol boils and takes the heat away, just try it by placing a drop of alcohol on your skin and blowing on it, you will feel your skin turn cold. (A motor recompresses the gas to a liquid and circulates it in the coils of the refrigerator; that is the sound you hear near the bottom of your fridge, and the reason why it is warm there.) The problem is alcohol is flammable, and other liquids they used like methyl chloride were toxic and odorless when they leaked. Often homes burned or people were poisoned. So a new gas needed to be found.
1n 1926 Thomas Midgley solved this problem by using a gas that has two chlorine atoms and two fluorine atoms bound to a carbon atom (dichlorodifluoromethane, also known as CFCs). This gas was perfect! Midgely even inhaled it and blew out a candle to show how it was non-toxic and it was non-flammable. Soon all refrigerators used this gas and food preservation became as normal as it is today. New uses were found for this gas in the propellants for aerosol cans and pesticide distribution during WWII. Production went unchecked for over fifty years! Until a couple of chemistry professors, Molina and Rowland asked a very simple question. What happens to all the CFC's that leak out of the old refrigerators that are discarded and not built well? This is a lot of gas. Think about the millions of people using these systems in homes, offices, cars, and in all the household propellants and commercial food refrigerators across the world.
Well the answer to that question is why I am down here. And it has to do with Ozone.
Today we prepared for a launch early in the morning. I spent hours on end calibrating instruments. The last balloon GPS locator failed, which means it will be an Easter Egg hunt trying to find it in October, using just a radio locator beacon.
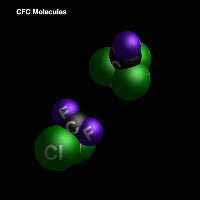
A chemist's view of CFC

Who is this?

Jenn working on Ozone Sonde calibration in Crary Lab
Contact the TEA in the field at
.
If you cannot connect through your browser, copy the
TEA's e-mail address in the "To:" line of
your favorite e-mail package.
|
