|
Frozen Water Before?
I'm Sure You Have; but I Bet You've Never Frozen Salt Water! Water !!!!!!!!
data
| hook
| main
| background & resources
| student
Hook
The investigate the freezing of salt water in the lab and compare
it to be the freezing of ocean water (sea ice)
Materials
- conductivity meter
- 2plastic tube 1" diameter, thin walled and 8"long
- permission from local supermarket for you (and several students) to use
their frozen food freezer
- make up 35 ppt (parts per thousand) of salt-water solution.
- graduate cylinder
- balance
- saw
- plastic bags for samples
- ruler
- pipette
- weighing cup
- salt solution (35 grams of salt/1000ml of water)
- construction insulation R =11 value (3/12"), 3" length
Procedure
- Wrap three layers of R11 insulation around full length of 8"
plastic pipe.
- stopper plastic tube , fill with 35 ppt salt water and stand
vertically in -20°C freezer till frozen
- when frozen (minimum of 24 hours) cut into 12" sections and place
in separate freezer bags marked so that location from "ice core" is known.
See the figures shown below for proper set up.
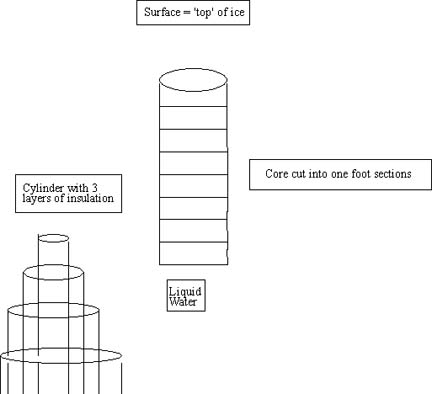
- allow melting at room temperature
- test each sample with conductivity meter to determine salt
concentration. (Make sure conductivity meter is standardized. Keep probe
in distilled water. Test sample, record conductivity and record reading.)
- mass weighing cup and using pipette, pipette out 4 ml of
solution; and determine its mass.
- calculate mass of solution in cup. (Subtract mass of cup from cup
plus sample = mass of sample.)
- calculate density of solution in cup. (Divide mass of sample by 4
ml. This will give the density in grams/ml.)
- repeat steps 4 - 7 with each of the sample from the ice core
- graph density (g/ml) as a function of where in ice core sample came
from "depth of ice" and graph "depth of ice" where in ice core sample came
from as a function of conductivity
Discussions Questions/Extensions ......
What are the differences between the freezing pattern (density and salt
concentrations gradients)of salt water in the lab and when "real" sea ice
forms?
Why is there a difference between the freezing pattern (density and salt
concentrations gradients)of salt water in the lab and when "real" sea ice
forms?
Return to top of page
Back to: TEA Activities Page
data
| hook
| main
| background & resources
| student
|


