11 November, 1999
Well, here we go again. I am scheduled to go out on the helo to Cape Roberts
this afternoon. The weather now is pretty nice (See image below), but the
forecast for this evening is much less so:
REGIONAL WEATHER SUMMARY: A low pressure system located off the Siple Coast
moving north towards the Ross Sea is producing yet another wave of
cloudiness and precipitation into the McMurdo area for this afternoon and
this evening.
TONIGHT: Mostly cloudy.
Visibility (nm): Reducing to less than ½ mile in snow/blowing snow.
Wind (knots): Southerly at 25-35 with gusts to 50.
Low: -08C/+18F. Lowest Wind-chill:
-30C/-23F.
Wish me luck! Again, if you don't see a journal tomorrow, you'll know I made
it.
I set up the evaporation experiment today. I hope you did too. I was a
little late with this because of some technical problems I had which I will
discuss later. The average initial depth of the water in my pan on 11/11/99
at 1:00PM NZT was 26mm. I will give you updates of the depth and water
temperature every couple of days.
When we start an experiment, it is a good idea to have some notion as to
what you expect to happen, a working hypothesis. Let's assume that the water
in the pan will, in fact, evaporate. We can also guess that my water may
evaporate at a different rate then yours. This doesn't mean that you won't
be alert for other results, but we need to have a focus of our observations.
Consider the following questions:
1. Do you think the water will evaporate?
2. If so, will your water and mine in Antarctica evaporate at the same rate?
3. What are the factors (variables) which control the rate of evaporation?
4. Which of those variables, if any, are we keeping constant?
5. Which variable or variables are different between your experiment and
mine? (Experimental or Dependent Variable)
6. What are we measuring the change in? (Independent Variable)
7. How will we be able to confirm (or refute) our hypothesis that the rate
at which my water evaporates is different then yours?
If you would like to try this experiment with me, and you do not have a copy
of it, e-mail me and I will send you a copy. (Microsoft Word document)
When I first set up a trial run of this experiment, I had to work out some
problems. First, the pie plates I brought with me were quite bent up from
the travel. I had a hard time getting the bottom of the pan flat. So I
devised the following method of determining the depth of the water. I took
the ruler and pushed it down as far as I could so the bottom of the pan was
pressed against the table. Also, I took three depth measurements. I measured
on the edge, in the middle and on the opposite edge. I then took the average
value of the three to report the depth.
However, after I ran the trial experiment for about 6 days, I ran
into an even more serious and interesting problem. There were about 20 small
pinholes in the bottom and sides of my aluminum pie plate. The water was
seeping out. Also, I observed around each hole a small amount of white solid
precipitate. Apparently, the tap water had eaten a hole through the pan in a
period of less than a week! This was quite intriguing. After all, this is
the water I am drinking each day. I plan on investigate this. (A note to my
AP Chemistry students: Perhaps you could give this some thought as well. You
might want to go back and look at my description of how the water
desalination process works here at McMurdo. See the November 9th journal. If
you have a possible solution as to why I have holes in the pie pan, e-mail
them to me.)
This is often the case in science. You start off doing an experiment
designed to solve one parcticular problem, and you run headlong into another
equally interesting problem. I will let you know if I find out anything
more.
Today's featured CRP Team Members are the two paleontologists who study the
fossil diatoms in the core samples. They are Dave Harwood from Lincoln,
Nebraska and Steve Bohaty from Wahoo, Nebraska. Both work at the University
of Nebraska. Diatoms are minute unicellular or colonial algae plankton with
skeletons formed from silicates. As with most of the paleontologists working
on the project, Dave and Steve are trying to use the occurrence of different
species of diatoms to help determine the age of the rocks and the
environment they were deposited. These types of paleontologists are called
biostratigraphers.

These are the current conditions as they appear on the TVs around town.

Here is my evaporation experimental set-up. The one on the right is covered with clear plastic wrap.

I press down firmly on the bottom of the pan with the ruler when I take the depth measurement.
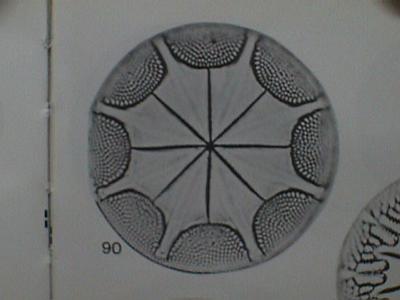
Here is a drawing of what a diatom looks like under a microscope. This parcticular one is called Astrolampra.

The Diatomicians! Dave Harwood, left and Steve Bohaty at the microscope.
Contact the TEA in the field at
.
If you cannot connect through your browser, copy the
TEA's e-mail address in the "To:" line of
your favorite e-mail package.
|
