

 Marge Porter is a teacher at Woodstock Academy in Woodstock, Connecticut.
She teaches environmental science and biology, as well as an introductory
general science course.
Marge Porter is a teacher at Woodstock Academy in Woodstock, Connecticut.
She teaches environmental science and biology, as well as an introductory
general science course.

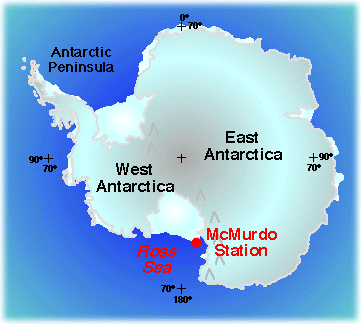
Properties of Sea Ice in Ross Sea, Antarctica
Martin Jeffries, University of Alaska, Fairbanks, Alaska
As a TEA, I parcticipated in a cruise onboard the Research Vessel Ice Breaker Nathaniel B. Palmer as part of a research team studying the properties of Antarctic sea ice. I found out there is a lot of variability in ice! It can grow in several different ways, each of which imparts a distinct structure to the ice. Sea ice also has a wide range in its salt content.
Structural variability in sea ice is an important variable in the heat budget of the upper ocean. Where sea ice is present, it acts as an insulator, greatly slowing the loss of heat from the relatively warm ocean to the much colder atmosphere. Different forms of ice vary in their insulating power, so it is important to know how much of each type is present. Sea ice is important to the overall energy balance of the Earth because it covers so much of the ocean in winter. In Antarctica, the summertime sea ice extent is about 4 million square kilometers (1.5 million square miles). Its maximum winter extent is five times greater, at around 20 million square kilometers (7.7 million square miles, or over twice as big as the United States!). That's a lot of ice!
We looked at three different forms of sea ice: frazil, platelet, and congelation ice. Frazil ice is made up of small crystals that form as sea water just begins to freeze. Turbulence in the water causes them to clump together in random orientations. Platelet ice forms in calm water deeper in the water column. These crystals look like thin plates. After they form, they float up and accumulate under the sea ice, forming a loose, honey-comb-like layer. Congelation ice forms by freezing onto the bottom of ice already present. Because it freezes "in place" in quiet water, it has a highly regular structure. The amount of salt in the ice (its salinity) varies. As sea water freezes, salt does not fit into the structure of the ice crystal, but some salt commonly remains trapped in the ice in pockets of very saline water (brine). The amount of brine trapped in and between ice crystals depends on how fast the ice freezes and how old it is. Older ice has had more opportunity for the brine to drain out.
The researchers are parcticularly interested in mapping the geographical distribution of the ice types, how the ice warps and deforms as it is pushed together, and how the presence of ice affects large-scale heat exchange processes in the Southern Ocean. The data they collect will be compared to data acquired by satellites. This will better enable us to design good experiments using satellites to study the "big picture" of how sea ice processes link our atmosphere and ocean.